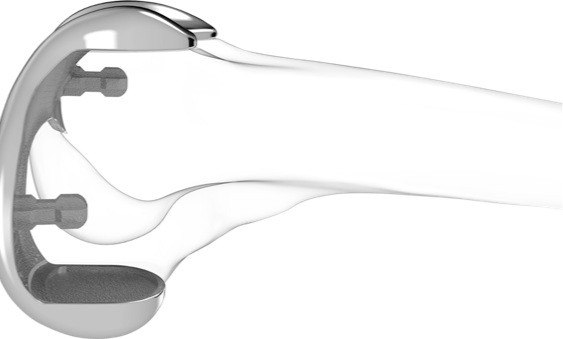
iTotal PS: Posterior Stabilized Total Knee Replacement
iTotal PS: The Only Truly Custom-Made Posterior Stabilized Total Knee Replacement
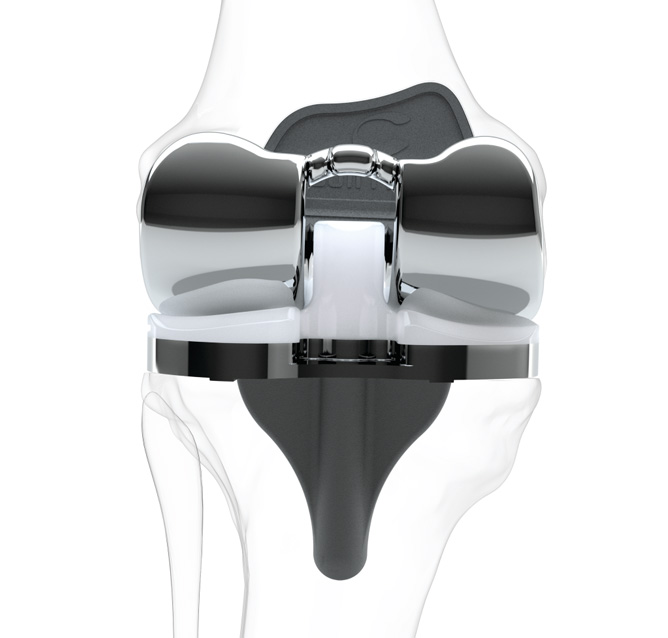
iTotal PS is the only truly custom-made posterior stabilized total knee replacement, designed with:
- Customized femoral and tibial fit to avoid overhang, under-coverage, and sizing compromises
- Customized shape designed to provide stability through range of motion and to restore kinematics
- Customized cam and spine to provide optimal stability and reduce potential for “mechanical” feel
- Patient data to enable a predictable, reproducible knee replacement surgery procedure
- A simplified delivery model that includes a single pre-sterilized kit, a single reusable instrument tray, and no implant inventory
- iTotal PS is designed with all the elements of the iTotal CR plus patient-specific cam and spine features to reduce mechanical issues that can cause component wear and impingement.
Other Conformis Products
Conformis also offers a cruciate retaining total knee option, as well as unicompartmental and bicompartmental implants.
Custom-Made Knee Implants
If it's not Conformis, it's not truly patient-specific
Custom-Made Knee Implants


 iTotal PS Brochure
iTotal PS Brochure