What to Expect from a Personalized Knee or Hip Replacement
Our personalized knee and hip replacement implants are designed to help restore your mobility and natural motion. Learn more about what to expect after knee replacement surgery below!
Knee Replacements Hip Replacements
What To Expect Before, During, & After Knee Replacement Surgery


“Today I can do everything I need to do without thinking about my knee."
What Happens Before Knee Surgery?

Getting Your CT Scan

Designing Your Knee Implant

Insurance Coverage for Knee Replacement
 SEE ALL PRIOR TO SURGERY KNEE REPLACEMENT FAQS
SEE ALL PRIOR TO SURGERY KNEE REPLACEMENT FAQS
Find A Doctor
Enter your zip code to find a doctor in your area.


What Happens During Knee Replacement Surgery?

How Long is Knee Replacement Surgery?

What Happens During Knee Replacement Surgery?

About Conformis Knee Implants
 Learn More About Our Implants
Learn More About Our Implants


"I’m doing things like row, weights, things I haven’t done in years.”
What Happens After Knee Replacement Surgery?

Knee Pain After Surgery

Physical Therapy After Knee Replacement

Going Back To Work
 SEE Real patient stories
SEE Real patient stories
We Asked: How Likely Are You Recommend Your Knee Replacement To A Friend Or Colleague?
% of Patients Selecting 10, Extremely Likely
The results of a national (U.S.) study by Precision Effect and SHG found that the vast majority of Conformis patients surveyed are confident and satisfied with their knee replacement, and are highly likely to recommend the knee to a friend or colleague.
 View The Full Study
View The Full Study
Frequently Asked Questions
Get answers to frequently asked questions about knee & hip replacement recovery time, and more from Conformis.
Watch our video resources to hear from
patients, surgeons, and learn about Conformis.
start with


Real Patients Stories
The Conformis experience is best described in our patients’ own words. Watch these knee replacement video testimonials from real Conformis patients to learn more about their surgery and recovery experiences.
 SEE Patient Experiences
SEE Patient Experiences 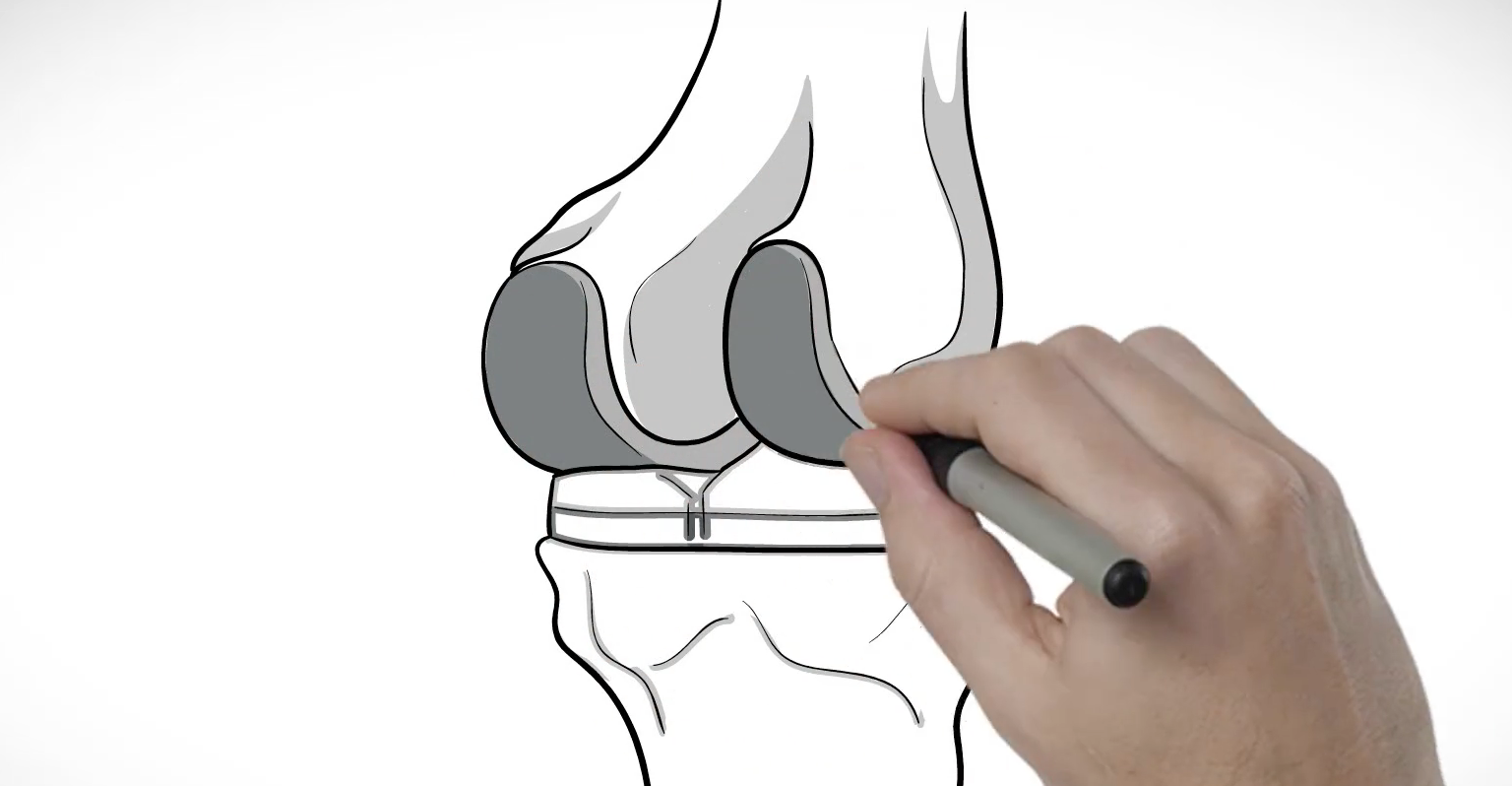
Learn About The Conformis Difference
We believe that everyone involved in the process of considering or receiving a joint replacement should be informed and aware of their options.
 SEE MORE ABOUT CONFORMIS VIDEOS
SEE MORE ABOUT CONFORMIS VIDEOS 
Real Surgeon Experiences
Find out why surgeons choose Conformis customized knee replacements for their patients.
 SEE Surgeon Experiences
SEE Surgeon Experiences The only truly patient-specific
total knee replacement
Conformis knee replacements are designed to match every aspect of your natural knee. The goal of any knee replacement is to be pain-free, restore natural motion, and for patients to return to their everyday activities.
 SEE MORE ABOUT KNEE REPLACEMENTS
SEE MORE ABOUT KNEE REPLACEMENTS
A new approach to
hip replacement
Cordera™ HipRx™ is the only primary total hip implant system on the market designed with 3D imaging technology to provide a femoral hip stem and acetabular cup size that matches each patient’s specific anatomy.
 SEE MORE ABOUT HIP REPLACEMENTS
SEE MORE ABOUT HIP REPLACEMENTS

